
Photo: Federick Ho from Flickr
1. Overview | 2. Morphology | 3. Organization of a Colony | 5. Kin Selection | 6. Communication & Foraging | 7. Distribution | 8. Taxonomy & Systematics | 9. References
1. Overview
Apis dorsata is the largest honey bee species in Singapore. Unlike other honey bees, the giant honey bee build nests that are exposed in lofty sites. Although being the largest species of the Apis, the giant honey bees do not tend to hide themselves as a large number of individuals can be seen forming a protective curtain around the nest at once. Colonies of giant honey bees have a strong tendency to aggregate on a single structure, creating a remarkably magnificent sight to behold. Nevertheless, it is always best to admire them from afar as colonies of A. dorsata are known to be aggressive, where up to three-quarters of the workers are engaged in colony defense.This page aims to educate one on the identification of A. dorsata, the social structure of honey bees, and the difference in behaviour of the giant honey bees from the dwarf and cavity-nesting honey bees. More interestingly, the conflict over species status of certain species in the subgenus Megapis will be discussed.
2. Morphology
2.1. Identification Key (Worker)
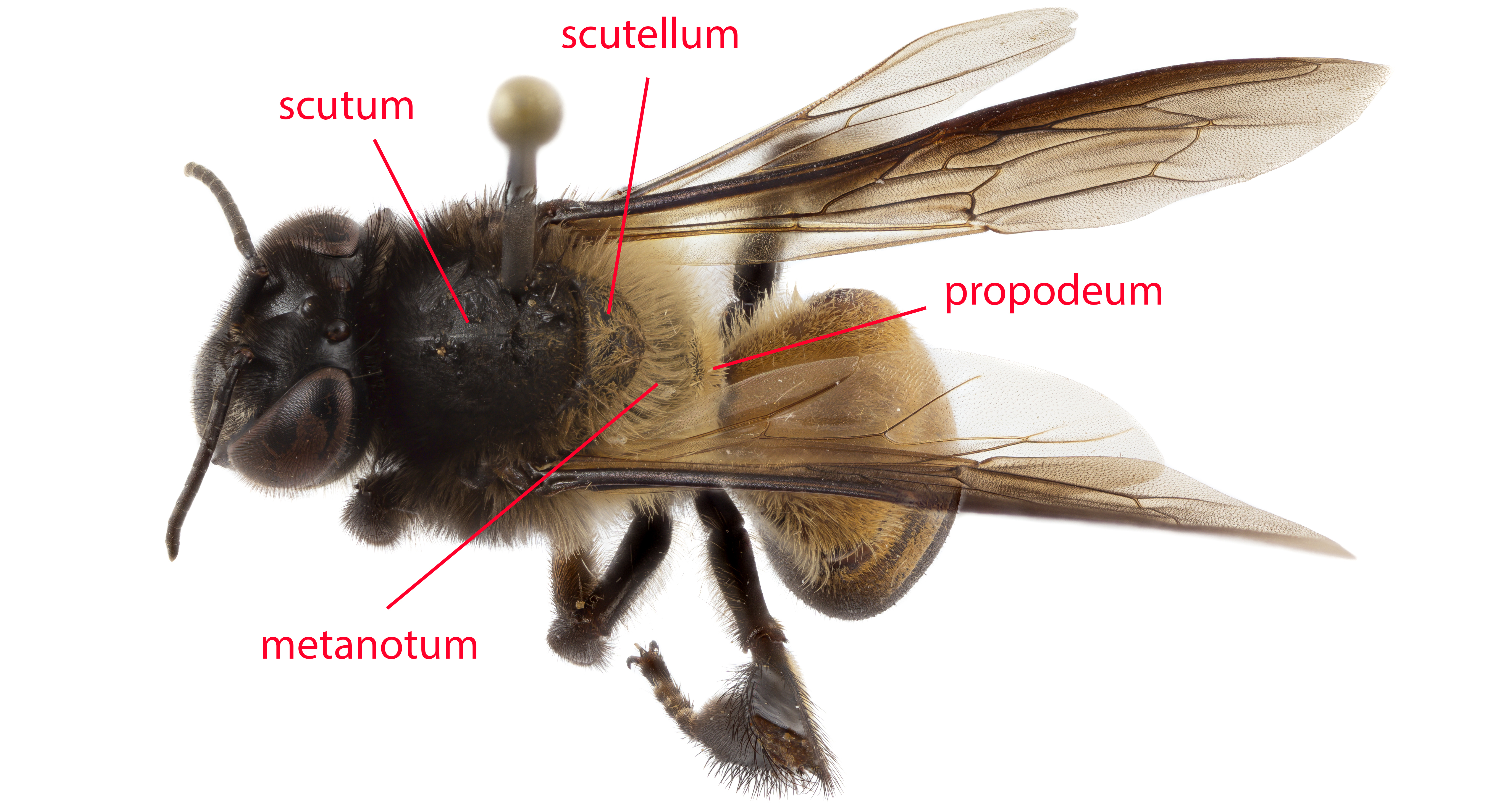
To spot a honey bee in general, one could look out for a black flying insect with black and yellow stripes on its abdomen. However, if visual identification is too difficult, one could always go to the extreme by catching the bee and getting stung by it - stinger of honey bee workers get dislodged (embedded in skin) after stinging. This is due to the barbed nature of the honey bee worker's stinger.The largest honey bee species in Singapore, A. dorsata can be easily differentiated from the other honey bee species by virtue of size (fig. 4). Another key identification to the A. dorsata is the contrasting hairs on the thorax. The scutum is covered with black hairs which contrast strongly with tan hairs of the scutellum, metanotum and propodeum (fig. 5).

A key to each species of Apis in Singapore was provided by J. S. Ascher:
 |
| Figure 6. A key to species of Apis in Singapore.Contributed by: J. S. Ascher |
3. Organization of a Colony
Each colony is made up of three castes: the queen, workers and drones. Castes are behaviourally, physiologically, and morphologically different to perform specific tasks. The caste system of honey bees results in an inevitable interdependence of all individuals. Each caste is irreplaceable and is vital for the survival of the colony.3.1. Queen
A colony normally has a single queen bee. Being the only individual of the female reproductive caste, the queen’s primary function is reproduction. The queen engages in nuptial flight one week after emergence from a queen cell to mate with multiple drones [1]. The adaptation to being the only egg-laying female relates to the queen’s greatly enlarged ovaries and spermatheca [2]. Other than reproduction, the queen also takes up the role of maintaining the organisation of her colony by producing pheromones using her mandibular glands [3][4][5][6][7].
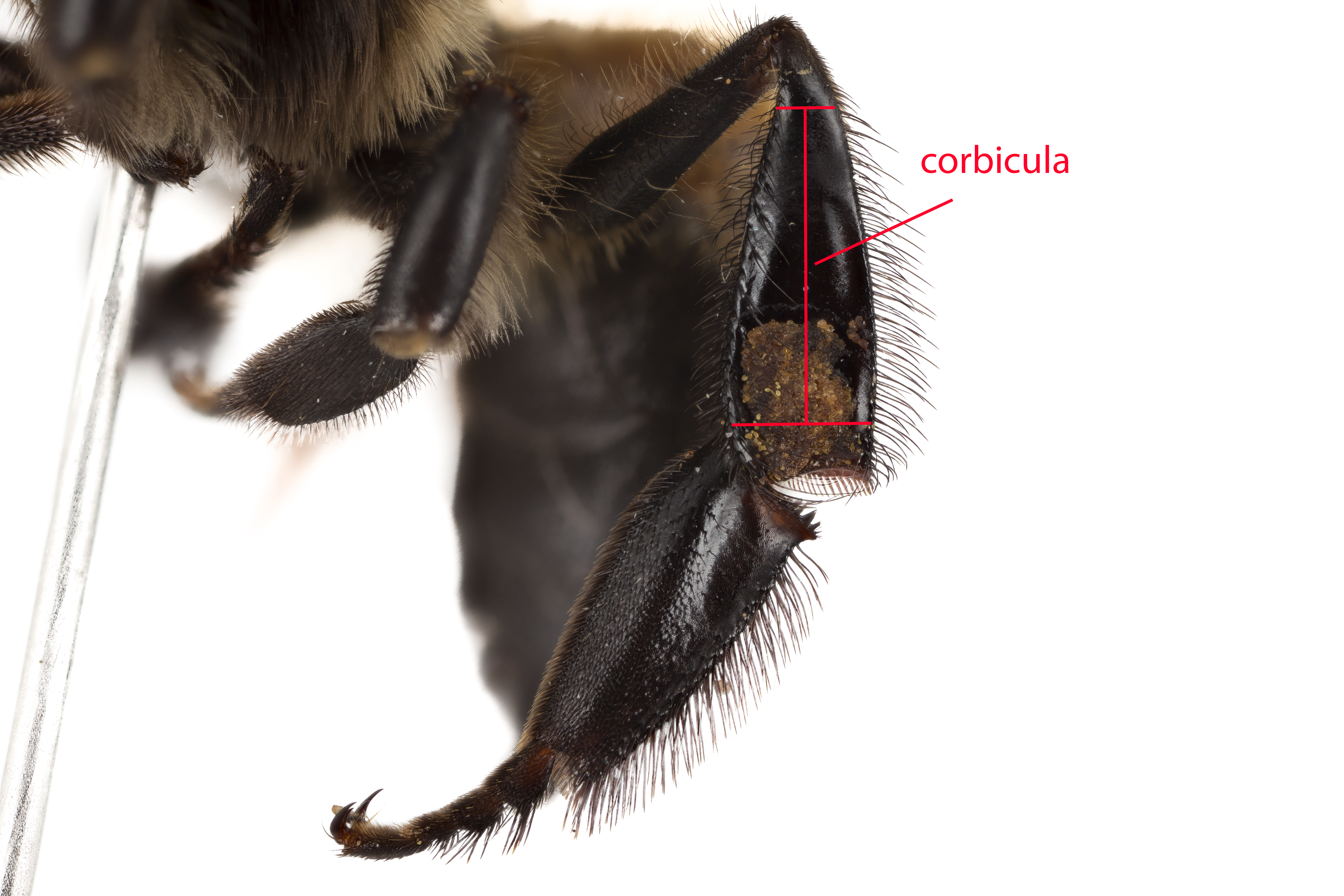
The queen can be easily distinguished from the workers and drones by a more tapered and wasp-like abdomen, and the lack of corbiculae [2]. Moreover, the sting of a queen is smooth, unlike the barbed sting of the workers (fig. 7). This allows the queen to sting other rival queens multiple times without dislodging her stinger along with her internal organs [2].
3.2. Worker
The worker population is made up entirely females. These females arise from the fertilized eggs (just like the queen), however are treated differently in terms of rearing conditions (refer to Section 4). Workers retain functional vestigial ovaries, but lack the full reproductive capacity of a queen and are effectively sterile. Although the workers are capable of producing fully functional male offspring, they actually gain more reproductive success by helping to rear their sisters rather than their offspring (refer to Section 5).Roles of the worker bees are age-specific and self-organizing [8]. The endocrine system of the worker changes over her lifetime to enable her to have the right toolkit to perform each task. The younger workers, having active hypopharangeal glands, perform hive-related tasks such as feeding the larvae and the queen. As they mature, their wax and poison glands become active, enabling them to build and guard the hive. The older workers forage for nectar and pollen. However, roles of workers can differ based on the needs of the hive.
Workers bees have modified parts that are not found in the queens. They possess the corbiculae (fig. 8) for gathering pollen, glands that produce wax or brood food, crop for nectar and barbed stings (fig. 7). These derived modifications are essential for the workers to perform their daily tasks.
3.3. Drone
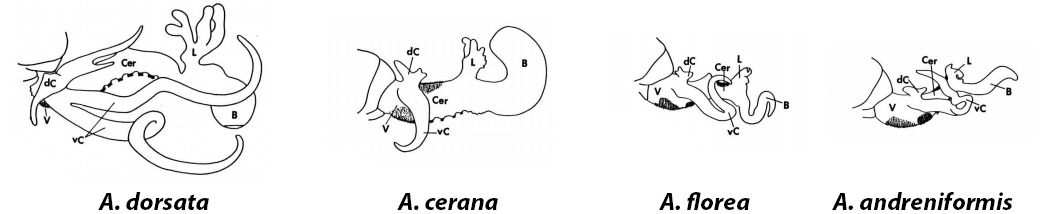
The drones make up the male reproductive caste and their sole purpose is to find mate. Drones are attracted to the sex pheromones produced by flying virgin queens [9]. Each mating flight can attract a swarm of up to 100 drones [10]. Although competition in mating with the virgin queens is high, no apparent sign of contest between the drones was observed [11].The honey bee penis, a large, fragile and membranous structure, is unique to its genus [12]. Each species of honey bee has their own unique male genitalia, which match the female genitalia like a ‘lock and key’ mechanism, to prevent interspecific mating (fig. 9) [13].
The video below shows how a drone grasps the queen’s thorax with his front legs and everts his penis into the queen sting chamber. The process of everting his penis instantaneously paralyzes the drone, causing it to dislodge its penis and falling away from the queen. The drone dies eventually.
Video: Mating flight of honey bees. Source: YouTube
Drones are easily recognizable by their huge eyes and short tongues (fig. 10).

4.1. Life Cycle
Apis dorsata share similar life cycle with the other honey bees. Honey bees are holometabolous insects which life cycle consists of four life stages – egg, larva, pupa and adult.The life cycle of a honey bee generally starts when a queen bee lays an egg in an individual brood cell. The time taken for an egg to develop into an adult differs between different castes (table 1) [15].
Table 1: Development time (days) of the brood of A. dorsata. Laval development means the time froman egg hatch to cell capping, pupal development the time from cell capping to adult emergence.

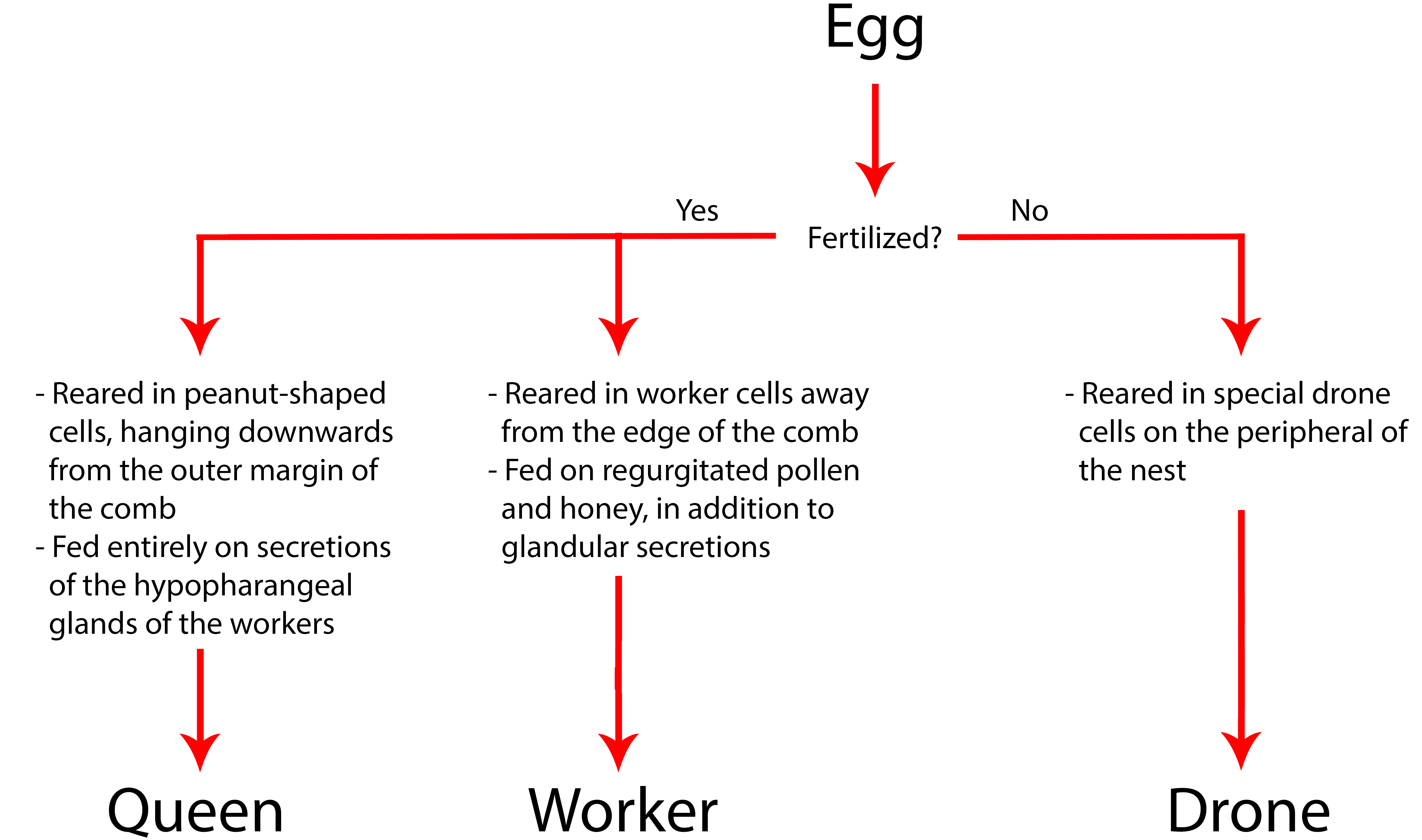
There are three different trajectories to how an individual egg can develop – a queen, a worker or a drone (fig. 14). The outcome of an egg is based solely on whether it has been fertilized, and its feeding and treatment as a larva.
4.2. Reproductive Swarm
Before the emergence of the new queens, the old queen and about half of the worker population take flight to establish a new colony elsewhere. Colonies of A. dorsata that are ready for reproductive swarm are seen to be characterized by a “cone” of young bees hanging down from the lower edge of the nest [16].4.3. Nest
The nest of A. dorsata is easily distinguished apart from the other three Apis in Singapore. Combs are massive (up to 1.5m wide and 1m long) and are built beneath a branch or overhang (fig. 15). A. andreniformis and A. florea build their combs around small branches while A. cerana build their combs in cavities.
4.4. Nest Defence
Nests consist of highly nutritious food source and valuable broods, and it is critical for A. dorsata to defend their hives from predators. By building their nests in lofty sites, A. dorsata are able to deter terrestrial predators. However, they are still susceptible to attack from flying organisms such as hornets and birds. A variety of defense mechanisms are employed to deter these predators.Shimmering behaviour is one of the defense mechanisms to deter flying insects, and sometimes birds [17]. The shimmer begins when an intruder approaches too near to the hive. Initially, a few workers will rapidly raise (towards the intruder) and lower their abdomens, often accompanied with a ‘buzz’ made by a single wing beat. This act then passes on to the adjacent workers, leading to a form of ripple across the surface of the hive (imagine a “kallang wave”). This behaviour tends to startle the predator, causing it to change its direction of flight.
Video: Shimmering behaviour of A. dorsata against hornetSource: YouTube
A. dorsata is known to be one of the most aggressive stinging insects [18]. The approach of a large organism within 10m of their colony triggers a defense mechanism where workers loosen their protective curtain which ‘enlarges’ the comb size [2]. This also allows many workers (400-600 workers in a second) to unleash an attack on the predator simultaneously if threatened further [19].
Although the defense mechanisms of A. dorsata may seems intimidating, there are predators that are still able to acquire the sweet, nutritious rewards within the hives.

5. Kin Selection
5.1. Haplodiploidy
Apis, like other aculeate Hymenopteras, has a unique genetic system called haplodiploidy – males are haploid and females are diploid (fig. 11). This allows the female reproductive caste to fully control the sex of her offspring by deciding whether the eggs are to be fertilized – a fertilized egg is diploid and, thus, develops into a female while an unfertilized egg develops into a male. Haplodiploid species live in an interesting social structure where the queens are surrounded by sterile workers that help to raise the young.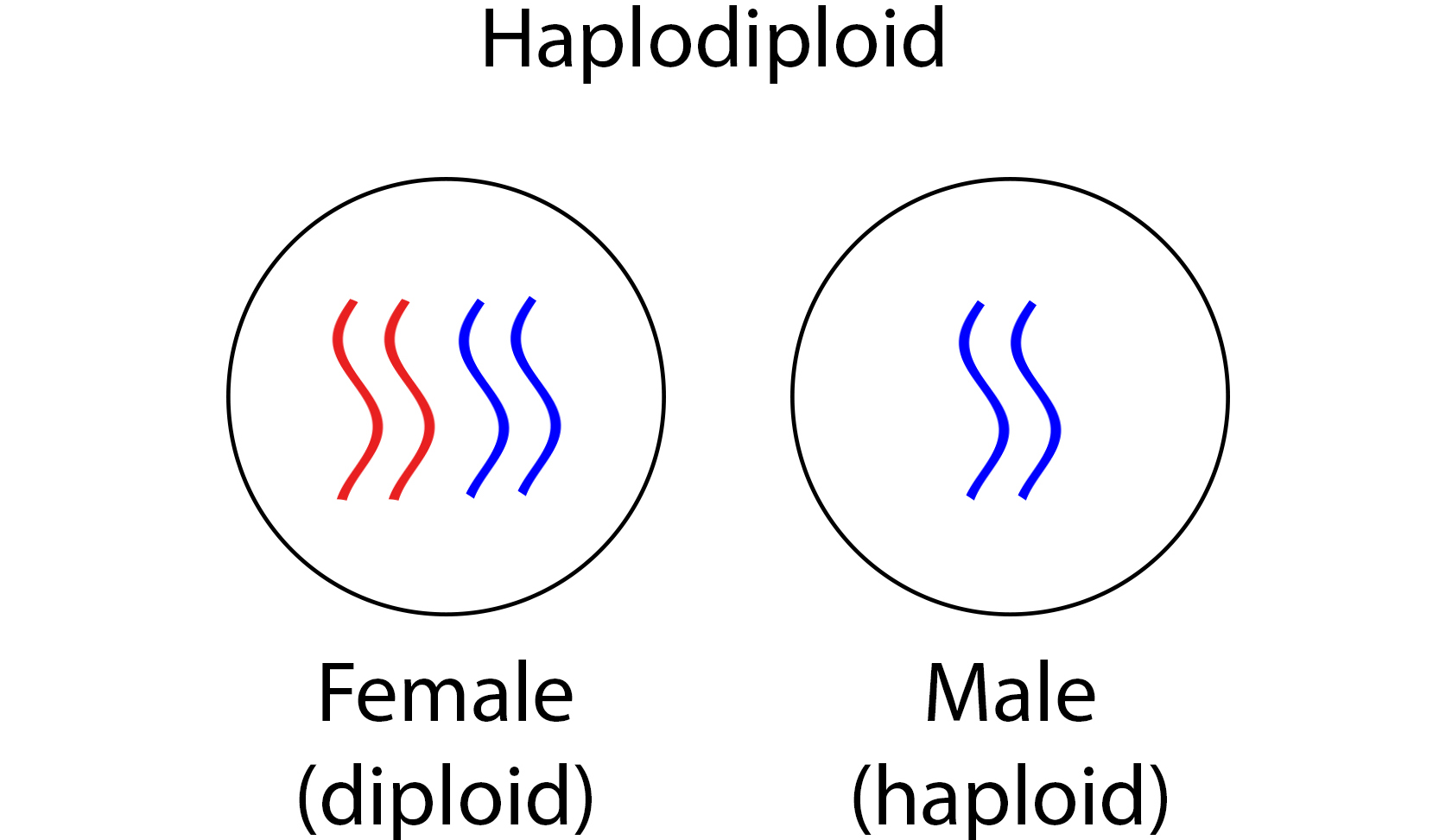
Haplodiploidy creates “supersister” where sterile workers are more closely related to the female offspring (supersisters) of their mother than their own daughters. Workers share 75% of their genetic materials (25% from the mother and 50% from the father) among themselves while only sharing 50% of their genetic materials with their own offspring (fig. 12). Thus, the workers gain more reproductive success by refraining from personal reproduction of daughters and helping to raise their supersisters. However, reproduction of male offspring by workers are often tolerated as workers are more related to each other (r=0.75) and their male offspring (r=0.5) than to the queen’s sons (r=0.25).
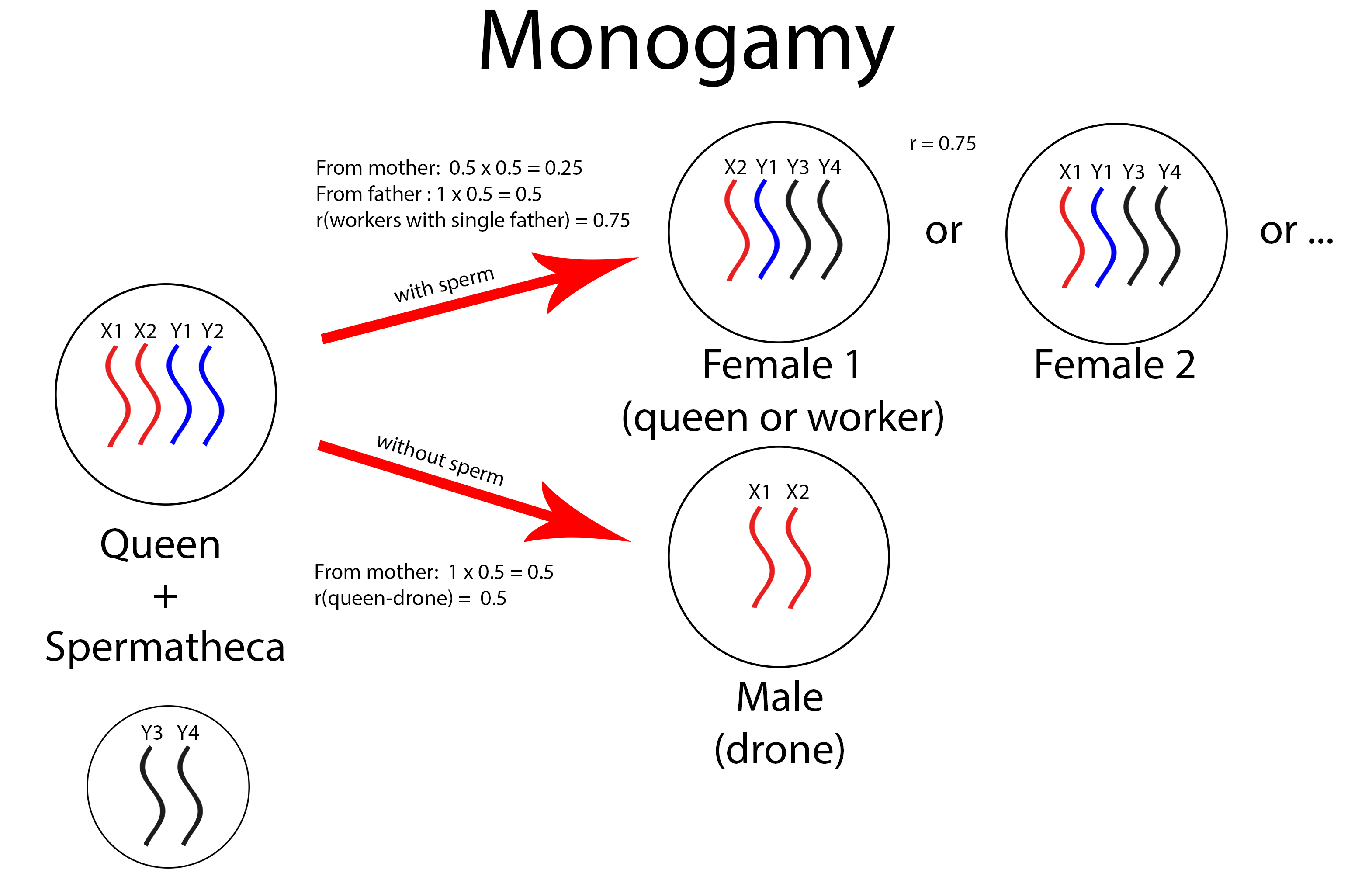
5.2. Polyandry & Worker Policing
In a polyandrous haplodiploid species like the honey bees, where queen mates with several males in one mating flight, the queens produce workers with asymmetrical relatedness. The worker population is made up of groups of half-sisters that are divided by their paternities (fig. 13). Workers of the same paternity are supersisters while workers of different paternities are half-sisters. This asymmetrical relatedness of the workers brings about worker policing where workers are favoured to suppress reproduction by other workers of different paternities [14]. Workers are more related to the queen’s sons (r=0.25) than to another worker’s sons (r=0.125). Ultimately, the queen will be the only one with reproductive success.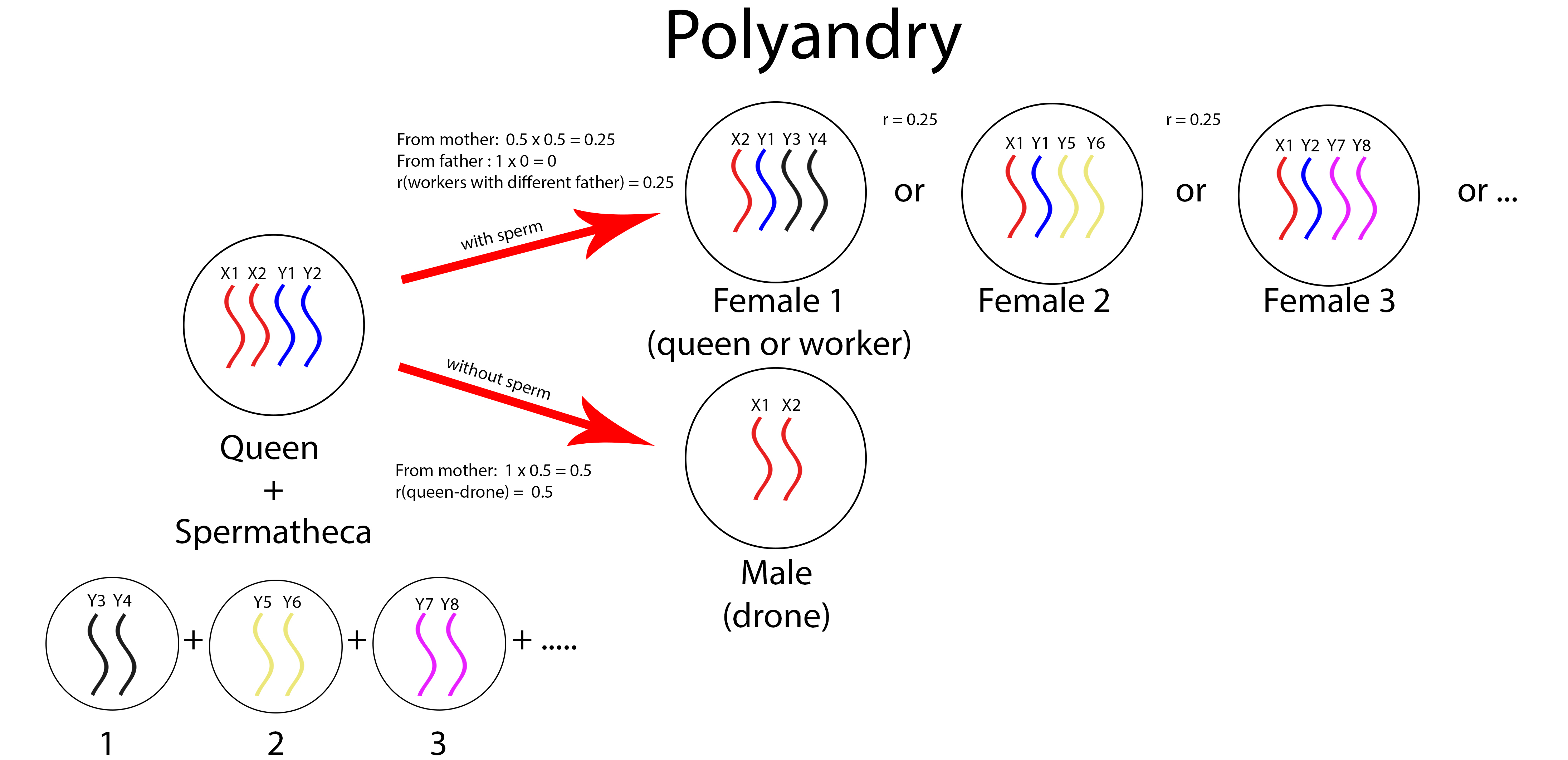
6. Communication & Foraging
6.1. Waggle Dance
Honey bees have an interesting way to communicate and convey information on productive foraging sites – dance! The dance runs in a figure-of-eight pattern (fig. 17). The honey bee starts off by striding forward while waggling her body side to side. This is termed the ‘waggle run’. At the end of the waggle run, the bee will make a sharp turn to either the left or right and return to the starting position to retrace the waggle run. At the end of the second waggle run, she will make another sharp turn to the opposite direction and return to the starting position. Thus, with every two waggle runs, the bee has gone through a figure of eight.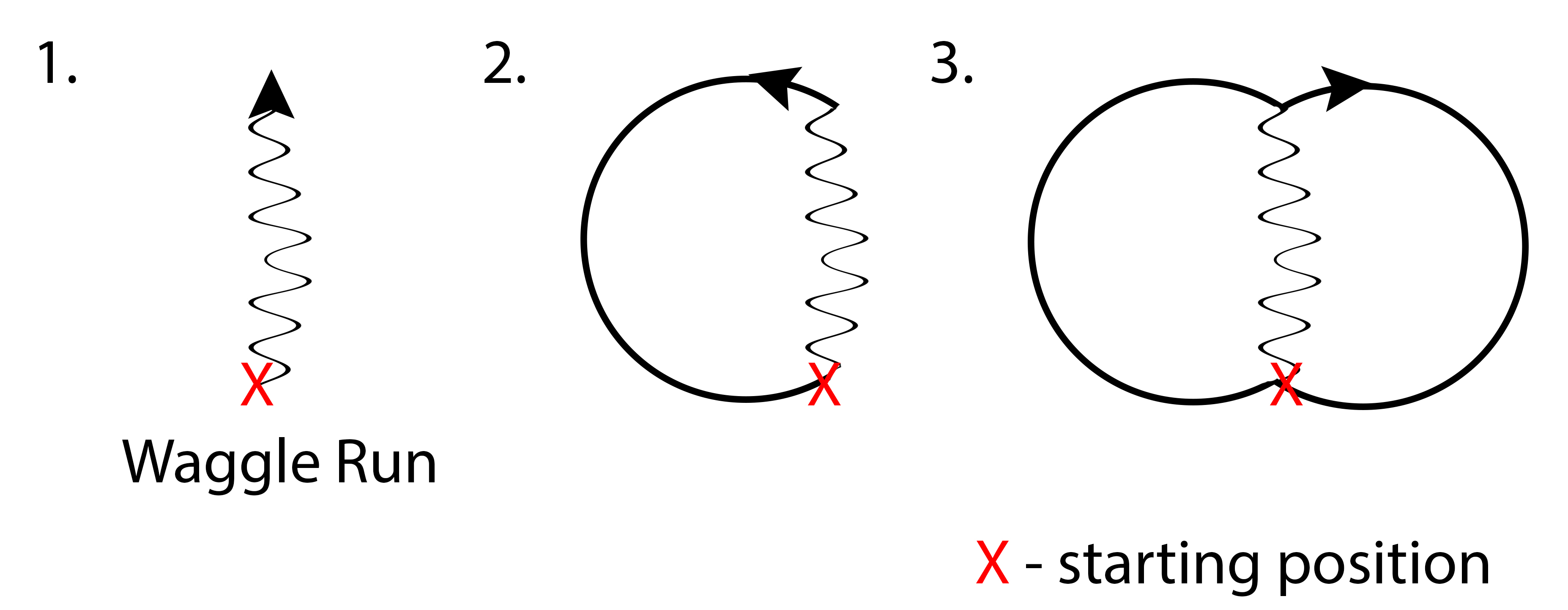
The waggle dance codes information such as distance, direction and richness of a foraging site. Distance to the site is through the duration of the dance, while richness is by the excitement of the dance [20]. The way each group of honey bees conveys the direction to the foraging site differ due to difference in location of the dance floor.
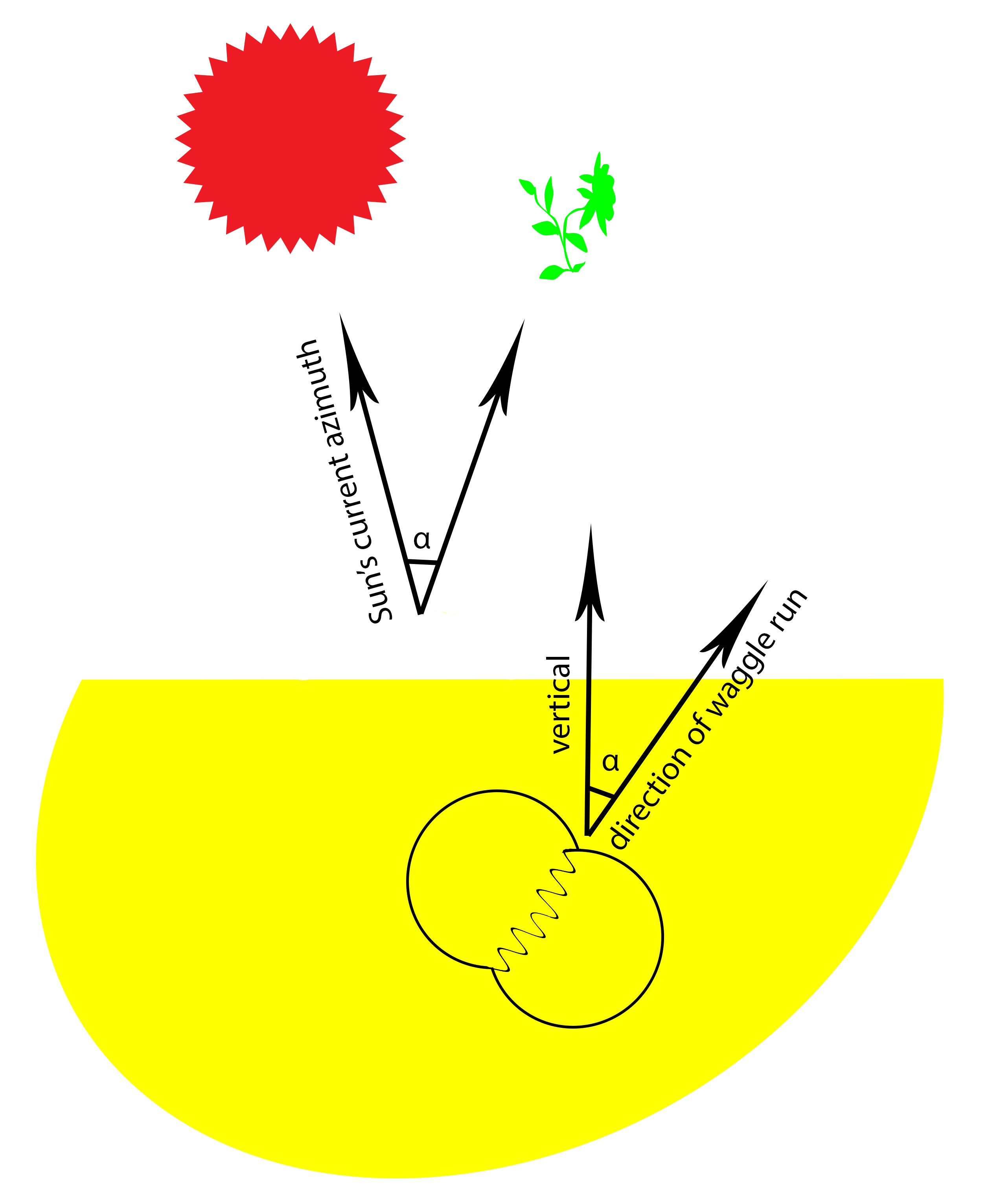
The dance floor, where the waggle dance is performed, of A. dorsata is located on the vertical plane of the comb. In order to convey the direction, the forager has to first estimate the angle deviation of the Sun’s current azimuth to the food source. After which, the forager will perform a waggle dance where direction of the waggle run and the vertical will deviate to the same degree as the Sun’s azimuth to the food source (fig. 18) [21]. It has been observed that the waggle runs of A. dorsata are accompanied by wing buzz sounds [22][23][24].
7. Distribution
7.1. Global
A. dorsata has a widespread distribution throughout southern Asia (fig. 2).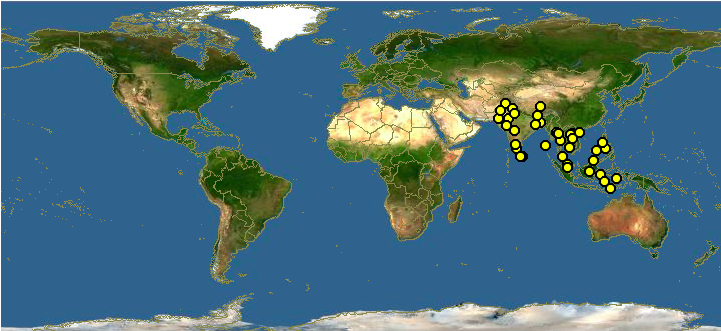 |
| Figure 2: Global distribution of A. dorsata. (with permission)Data & Map: Discover Life |
7.2. Local
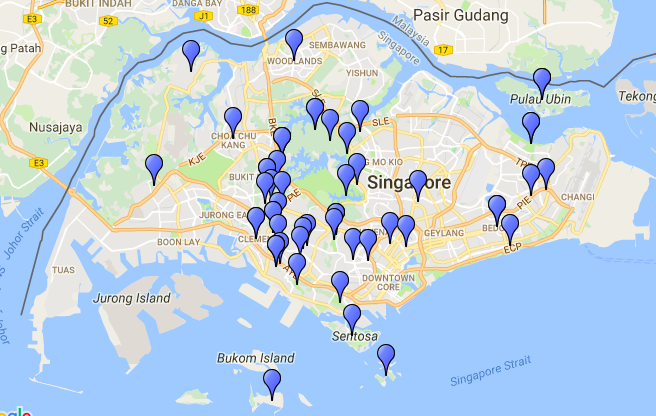 |
| Figure 3: Local distribution of A. dorsata. Data: NUS Bee Lab |
8. Taxonomy & Systematics
8.1. Classification
Kingdom: AnimaliaPhylum: Arthropoda
Class: Insecta
Order: Hymenoptera
Superfamily: Apoidea
Family: Apidae
Subfamily: Apinae
Tribe: Apini
Genus: Apis
Subgenus: Megapis
Species: dorsata
Data from ITIS
8.2. Type Information
Type locality: India orientaliType sex: Worker
Type repository: Natural History Museum of Denmark – Zoological Museum
8.3. Phylogeny
The phylogeny of the honey bees, through molecular, behavioural, and morphological data, has come to a consensus that the genus Apis is separated into three subgenera: Micrapis, Megapis & Apis. In figure 19, major clades were supported with high bootstrap values (fig. 19). It can be seen that open nesting is a more plausible basal character trait than cavity nesting. Cavity nesting being lost twice during evolution leading to the open nesting honey bees is less probable than cavity nesting being derived once.Although much studies have been done on Apis in general, there have been no comprehensive morphometric or DNA studies conducted on giant honey bees. Thus, species status within Megapis are suggestive.
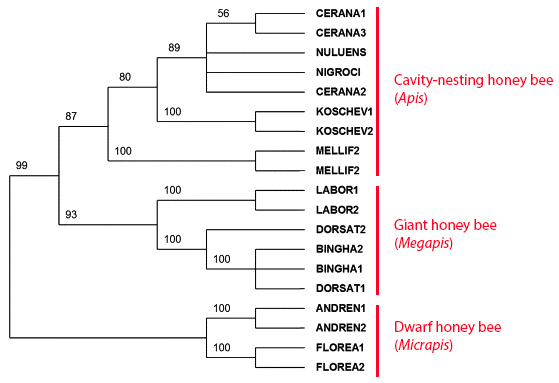
Fig 19: Bootstrap 50% majority-rule consensus tree of six equally parsimonious trees, from ND2 sequence data (361 steps, CI = 0.726, RI = 0.832). Bootstrap frequencies (2000 replicates) are indicated for each node.Adapted from Arias & Sheppard (2005)
In the subgenus Megapis, the widely debated argument would be the species status of A. laboriosa (author’s opinion; A. d. laboriosa would be used if regarded as subspecies) that is distinct from A. dorsata. Apis laboriosa, the Himalayan honey bee, is only found above 1500m in the Himalayas. It is obvious why many taxonomists do not recognize A. laboriosa as a species on its own (fig. 20). Other than the slight difference in overall size and the colour of the hair on the scutum, the two species of giant honey bees are morphologically similar [25].

There are many evidence suggesting species status of A. laboriosa:
1) Difference in time of mating flight – A. laboriosa takes flight between 12:30 and 14:30 [26] while A. dorsata takes flight just after dusk [27][28].
2) Difference in dance language – waggle run of A. dorsata is accompanied with wing buzz sounds while A. laboriosa does not [29].
3) There is a significant difference (10.6-11.5%) in DNA sequences of the two species [30].
4) In figure 19, samples of A. laboriosa form a unique well-defined cluster with high bootstrap value.
However, species status of A. laboriosa is still extensively discussed and more observations or DNA work studies have to be done.
As previously mentioned that species status are suggestive, below are two widely recognised subspecies of A. dorsata:
 |
 |
9. References
1 Tan, N. Q., M. Mardan, P. H. Thai, and P. H. Chinh. 1999. Observations on multiple mating flights of Apis dorsata queens. Apidologie. 30: 339-346.2 Oldroyd, B. P., and Wongsiri, S. 2006. Asian honey bees: biology, conservation, and human interactions. 7-8.
3 Free, J. B. 1987. Pheromones of social bees. Chapman and Hall: London.
4 Plettener, E., K. N. Slessor, M. L. Winston, G. E. Robinson, and R. E. Page. 1993. Mandibular gland components and ovarian development as measures of caste differentiation in the honey bee (Apis mellifera L.). Journal of Insect Physiology 39: 235-240.
5 Plettener, E., G. W. Otis, D. C. Wimalaratne, M. L. Winston, K. N. Slessor, T. Pankiw, and P. W. K. Punchihewa. 1997. Species- and caste-determined mandibular gland signals in honeybees (Apis). Journey of Chemical Ecology 23: 363-377.
6 Winston, M. L., and K. N. Slessor. 1998. Honey bee primer pheromones and colony organization: gaps in our knowledge. Apidologie 29: 81-95.
7 Wossler, T. C., and R. M. Crewe. 1999. Honeybee queen tergal gland secretion affects ovarian development in caged workers. Apidologie 30: 311-320.
8 Lindauer, M. 1967. Communication among social bees. Harvard University Press: Cambridge.
9 Gary, N. E. 1962. Chemical mating attractants in the queen honey bee. Science 136: 773-774.
10 Gries, M., and N. Koeniger. 1996. Straight forward to the queen: pursuing honeybee drones (Apis mellifera L.) adjust their body axis to the direction of the queen. Journal of Comparative Physiology A 179: 539-544.
11 Koeniger, G. 1990. The role of the mating sign in honey bees, Apis mellifera L.: does it hinder or promote multiple mating? Animal Behaviour 39: 444-449.
12 Ruttner, F. 1988. Biogeography and taxonomy of honeybees. Springer-Verlag: Berlin.
13 Koeniger, G., N. Koeniger, M. Mardan, G. Otis, and S. Wongsiri. 1991. Comparative anatomy of male genital organs in the genus Apis. Apidologie 22: 539-522.
http://dx.doi.org/10.1051/apido:19910507
14 Ratnieks, F. L. W. 1988. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. American Naturalist 132: 217-236.
15 Qayyum, H. A., and A. Nabi. 1968. Biology of Apis dorsata. Pakistan Journal of Science 19: 109-113.
16 Woyke, J., J. Wilde, and M. Wilde. 2000. Swarming, migrating, and absconding of Apis dosata colonies. In Seventh international conference on tropicalbees: management and diversity and fifth Asian Apicultural Association conference, Chiang Mai; Thailand, ed. S. Wongsiri, pp. 183-188. International Bee Research Association: Cardiff.
17 Seeley, T. D., R. H. Seeley, and P. Akratanakul. 1982. Colony defense strategies of the honeybees in Thailand. Ecological Monographs 52: 43-63.
18 Morse, R. A, and F. M. Laigo. 1969. Apis dorsata in the Philippines. Philippines Association of Entomologists: Laguna.
19 Kastberger, G., and D. K. Sharma. 2000. The predator-prey interaction between blue-bearded bee eaters (Nyctyornis athertoni Jardine and Selby 1830) and giant honey bees (Apis dorsata Fabricius 1798). Apidologie 31: 727-736.
20 Seeley, T. D., A. S. Mikheyev, and G. J. Pagano. 2000. Dancing bees tune both duration and rate of waggle-run production in relation to nectar source profitability. Journal of Comparative Physiology A 186: 813-819.
21 Lindauer, M. 1954. Temperaturregulierung und Wasserhaushalt im Bienenstaat. Zeitschrift fur Vergleichende Physiologie 36: 391-432.
22 Dreller, C., and W. H. Kirchner. 1994. Hearing in the Asian honeybees Apis dorsata and Apis florea. Insectes Sociaux 41: 291-299.
23 Kirchner, W. H. 1993. Acoustical communication in honeybees. Apidologie 24: 297-307.
24 Kirchner, W., and C. Dreller. 1993. Acoustical signals in the dance language of the giant honeybee, Apis dorsata. Behavioral Ecology and Sociobiology 33: 67-72.
25 Engel, M. S. 1999. The taxonomy of recent and fossil honey bees (Hymenoptera: Apidae; Apis). Journal of Hymenopteran Research 8: 165-196.
26 Underwood, B. A. 1990b. Time of drone flight in Apis laboriosa Smith in Nepal. Apidologie 21: 501-504.
27 Koeniger, N., and H. N. P. Wijayagunesekera. 1976. Time of drone flight in three Asian honeybee species (Apis cerana, Apis florea, Apis dorsata). Journal of Apicultural Research 15:67-71.2
28 Rinderer, T. E., B. P. Oldroyd, S. Wongsiri, S. Potichot, W. S. Sheppard, and S. Buchmann. 1993. Time of drone flight in four bee species in south-eastern Thailand. Journal of Apicultural Research 32: 27-33.
29 Kirchner, W. H., C. Dreller, A. Grasser, and D. Baidya. 1996. The silent dances of the Himalayan honeybee, Apis laboriosa. Apidologie 27: 331-339.
30 Arias, M. C., and W. S. Sheppard. 2005. Phylogenetic relationships of honey bees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution 37: 25-33.